

You need to lift your foot to the height of the step to move on. If you don't lift your foot enough, you will bump into the step and be stuck on the ground level. It is helpful to think of this like going up a flight of steps.
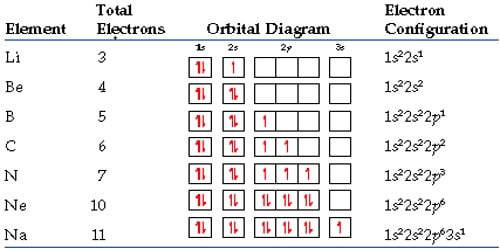
Each element emits light at a specific frequency (or color) upon heating that corresponds to the energy of the electronic excitation. One way an electron can release energy is by emitting light. For the excited electron to go back to its original energy, or ground state, it needs to release energy. When the energy of an atom is increased (for example, when a substance is heated), the energy of the electrons inside the atom is also increased-that is to say, the electrons get excited. Electrons that have higher energy are found further away. Electrons with the lowest energy are found closest to the nucleus, where the attractive force of the positively charged nucleus is the greatest. Although these electrons all have the same charge and the same mass, each electron in an atom has a different amount of energy. The central structure of an atom is the nucleus, which contains protons and neutrons. quantization: The process of approximating a continuous signal by a set of discrete symbols or integer values.frequency: The number of occurrences of a repeating event per unit of time.Electrons will separate as much as possible within a shell. Each orbital can hold only one electron pair. An electron will move to the orbital with lowest energy. There are guidelines for determining the electron configuration of an atom.An orbital diagram is used to determine an atom's electron configuration. Within each shell, the s subshell is at a lower energy than the p. There are four different orbital shapes: s, p, d, and f. In a more realistic model, electrons move in atomic orbitals, or subshells.An atom's electron shell can accommodate 2n 2 electrons (where n is the shell level). Electrons further away from the nucleus will have higher energy. Electrons closest to the nucleus will have the lowest energy. Viewed simply, electrons are arranged in shells around an atom's nucleus.The energy of the light released when an electron drops in energy level is the same as the difference in energy between the two levels. To go back to its ground state, the electron releases energy. If the energy of an atom is increased, an electron in the atom gets excited.


 0 kommentar(er)
0 kommentar(er)
